Novartis heart drug a China case study: How drug exclusivity dies by a thousand filings

Entresto, Novartis's blockbuster drug to treat chronic heart failure, lost its core US patent protection on July 16, opening the floodgates for global generics. China is already ahead in the race.
Often called the engine of global generic drugs, China produces nearly 80 percent of the active ingredients used in off-patent medications worldwide. Now it's stepping further up the chain.
At least 13 Chinese pharma firms have already won approval to sell their own versions of Entresto at home, with dozens more in clinical trials or waiting in line. Some are cutting licensing deals or challenging leftover patents to get an edge.
In truth, Entresto's legal shields in China began crumbling years go. China is the first major market where generics could legally launch, giving its pharmaceutical industry a head start. The question is no longer whether China can compete, but how far its generics makers are willing and able to go.
Novartis fights back
Entresto was Novartis' top-selling product in 2024, with sales of US$4.05 billion in the US and US$7.82 billion worldwide. The Swiss pharmaceutical giant says Entresto, since its 2015 launch, has reduced the risk of death and hospitalization for more than 2.5 million people with chronic heart failure.
That commercial success made Entresto a target. On July 16, a US district court denied Novartis' request to block India-based MSN Pharmaceuticals from launching a generic version, ruling that "the availability of a generic alternative to a life-saving medication is in the public interest."
Entresto is a prime example of what happens when patent exclusivity collapses, and a China case study in how quickly a drug can go from protected to besieged.
In 2018, Chinese generic drug makers launched a coordinated "assault" on Entresto's compound and crystal patents. One after another, challengers lined up – Shenzhen-based Salubris, CSPC Pharmaceutical, Nanjing-based Chiatai Tianqing. They weren't just filing applications; they were filing lawsuits.
By 2021, the Novartis patent on the cornerstone 2.5-hydrate crystal had been declared partially invalid. Novartis appealed the decision to China's top intellectual property court and lost.
That should have been checkmate, but the Swiss drugmaker didn't retreat; it regrouped.
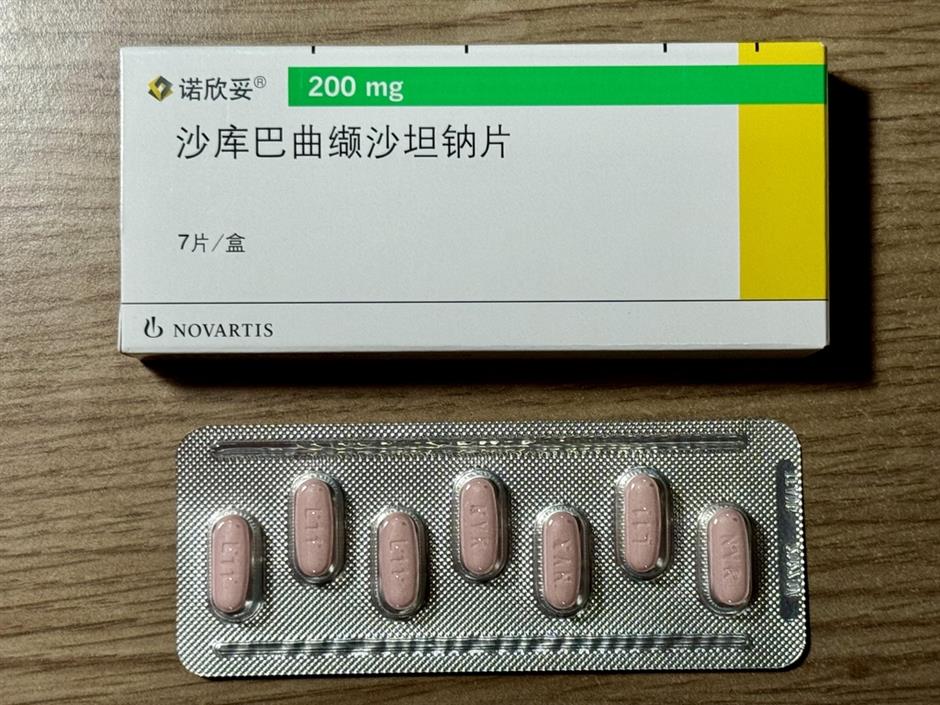
License, litigate, delay
First, it filed for patent term extensions, hoping to squeeze more time from whatever protection remained.
Then Novartis shifted its defenses to a lesser-known weapon: the "0-3 hydrate" crystal. Chiatai Tianqing obtained an implementation license for this crystal form but is still expected to wait until the 2.5-hydrate patent expires before it can launch its version.
Jiangsu Province-based Huahan also secured a license to produce Entresto under Novartis's authorization, giving it a rare early-mover advantage.
The battlefield kept expanding. Some challengers took an aggressive stance with a "Type 4.2 declaration," asserting that their products do not infringe existing patents – a risky move that can trigger a nine-month regulatory hold, but if successful, grant a 12-month exclusivity window.
Others, such as Changyao in Jiangsu Province and Qilu Pharmaceutical, chose "Type 3 declarations," acknowledging the patents and pledging to wait until expiry before marketing their products.
By late 2024, more than a dozen companies had already been approved to market their generics, another 21 were in the registration queue and 15 more were conducting bio-equivalence trials.
The gates had been breached, but other pharma counties were still camped outside, preparing to their own assaults.
Facing challengers on all fronts, Novartis began choosing its battles: cutting selective licensing deals, granting crystal-form permissions and deploying administrative maneuvers to delay the inevitable.
It worked – briefly. Legal feints and settlements bought time, just enough to hold market share for another quarter. But the walls had been irrevocably cracked.
Behind the breach, what the siege signals
This was not only a patent expiration but a precision strike. Chinese drugmakers didn't wait for the gates to crumble. They mapped out every flank, from compound to crystal to formulation. Some slipped through with Type 3 declarations, promising to wait. Others kicked the door with Type 4.2 filings, gambling for a head start and a 12-month prize. The more cautious drew up licensing deals. The boldest simply marched in.
"The system aims to strike a balance," said lawyer Zhang Qiulin. "If generics overwhelm originators, innovation dries up. If originators dominate unchecked, drugs become unaffordable. We need both good medicine and affordable access, so patients benefit in the end."
That balance, he added, is exactly what China's "patent linkage" system was designed to protect and not tilt the scales toward innovators or copycats. It aims to encourage innovation and higher-quality generics while safeguarding the public's access to safe and affordable medicine.
With that framework in place, the system hasn't slowed generic challengers. It gave them coordinates. What once was a legal minefield turned into a battlefield. Under China's patent linkage system, every generics player had to show its hand before entering. No more shadow play. Everyone picked a lane – declare, delay or dispute – and moved fast.
The next battles won't be about winning. They'll be about how fast you lose. In a war where patents expire in waves and exclusivity dies by a thousand filings, speed is the new advantage. And in China's generics playbook, delay is defeat.
In Case You Missed It...
![[Open Seasame] Yo... Expats CAN Use Community Centers – a Guide!](https://obj.shine.cn/files/2026/01/10/61e3bfc3-d4d2-4efd-aa2c-9a6859cecea1_0.jpeg)